iLINK™ CORNEAL CROSS-LINKING
The diagnosis of an eye disease known as keratoconus happens in one of every 2,000 people in the United States, although some estimates suggest the number is much higher, at one in every 400 people.
If you are one of the people with this diagnosis, you may benefit from iLink™ corneal cross-linking, the first and only FDA-approved procedure to slow or halt the progression of keratoconus disease.
What Is Keratoconus?
Keratoconus (KC) is a non-inflammatory eye disease. KC is a progressive condition where the typically dome shaped front of your eye, called the cornea, begins thinning and weakening. This causes a cone shape bulge to develop and can significantly impair your vision.
What Are the Symptoms of Keratoconus?
The symptoms of KC usually occur during your teens to early adulthood. The disease progressively worsens but in the early stages you may experience:
- Increased light sensitivity
- Slightly blurred vision
- Vision distortion
The cornea plays an important role in your ability to see by focusing the light that enters the eye. Any corneal abnormalities, but especially keratoconus, which can greatly affect how you see and interact with the world. Simple tasks, like driving a car or reading can be very difficult.
If left untreated, one in five keratoconus patients may require a corneal transplant. Given that most diagnoses of KC occur during the teenage years or early 20s, some patients may require more than one corneal transplant in their lifetime.
Can Keratoconus Be Prevented?
To determine if the disease can be prevented, we must first know the cause. Unfortunately, we still don’t know what causes keratoconus. These cases can occur randomly for reasons we don’t yet understand. However, KC does seem to have a genetic component. There also may be a connection to other underlying disorders such as asthma, Down syndrome, eczema, or sleep apnea.
Some studies suggest environmental factors may trigger the disorder in people who are genetically predisposed to the disease. Ultimately, scientists believe the disease stems from a complex mix of both environmental and genetic factors.
Keratoconus Facts:
- KC can result in significant vision loss
- In severe cases, KC may lead to corneal transplant
- 10% of people with KC have relatives affected by the diseases
- People with Down syndrome are 20 times more likely to be affected with KC
What Is the Best Treatment for Keratoconus?
iLink™ corneal cross-linking is a minimally invasive outpatient procedure that combines the use of ultraviolet light and specially formulated eye drops to stiffen and strengthen corneas weakened by KC disease or refractive surgery.
Cross-linking is considered the standard of care around the world for progressive keratoconus and corneal ectasia that follows as a complication of refractive surgery.
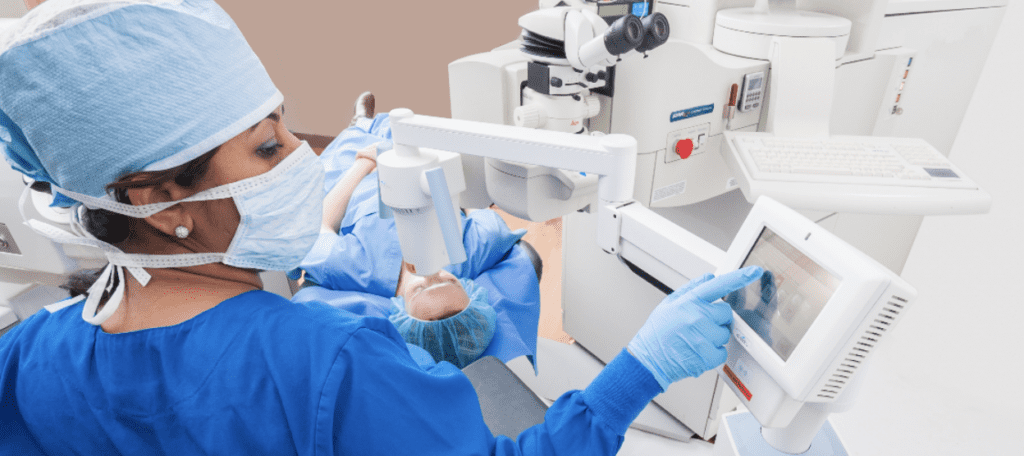
How Does iLink™ Corneal Cross-Linking Work?
iLink™ corneal cross-linking uses two specially formulated prescription eye drops to treat keratoconus:
- Photrexa® (riboflavin 5′-phosphate ophthalmic solution)
- Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution)
The iLink™ corneal cross-linking procedure works in two steps:
- The cornea will be saturated with these eye drop medications. Riboflavin is a type of B vitamin that allows the corneal tissue to absorb ultraviolet light.
- Ultraviolet (UV) light from a machine called the KXL® System will halt the progression of keratoconus.
This process works by creating new, stronger collagen cross-links to strengthen and stiffen the cornea. It’s a painless, non-invasive, but highly effective treatment for your KC.
iLink™ is the only FDA-approved corneal cross-linking procedure in the U.S. Rigorous clinical studies show this treatment is both safe and effective at stopping or slowing the progression of keratoconus.
Is iLink™ Corneal Cross-Linking Covered by Insurance?
iLink™ corneal cross-linking is widely recognized and now covered by more than 95% of the insurance plans in the United States. In fact, iLink™ is the only corneal cross-linking procedure recognized and reimbursed by all the major insurance carriers today.
All keratoconus treatments are not the same. Only iLink™ is both FDA-approved and covered by insurance.
Is iLink™ Corneal Cross-Linking Right for You?
Patients who have been diagnosed with progressive KC or corneal ectasia following refractive surgery should ask their doctor about iLink™ corneal cross-linking.
Don’t risk your eyesight on a procedure or product that does not offer full safety and efficacy as approved by the FDA. If your KC isn’t effectively treated, the vision that you lose as the disease progresses cannot be recovered.
At Your Eye Specialists, we are proud to be the only practice between Dade and Palm Beach to offer the iLink™ corneal cross-linking technology to help patients stop the progression of their KC and retain their vision longterm. Contact us today to learn more about your options.
What People Are Saying About Us
Trust Your Vision to South Florida’s Premier Eye Doctors
Saying YES is all it takes!


